CMS117v7 – Childhood Immunization Status
| Childhood Immunization Status | CMS117v7 | Percentage of children 2 years of age who had four diphtheria, tetanus and acellular pertussis (DTaP); three polio (IPV), one measles, mumps and rubella (MMR); three H influenza type B (HiB); three hepatitis B (Hep B); one chicken pox (VZV); four pneumococcal conjugate (PCV); one hepatitis A (Hep A); two or three rotavirus (RV); and two influenza (flu) vaccines by their second birthday |
|---|---|---|
| – DENOMINATOR
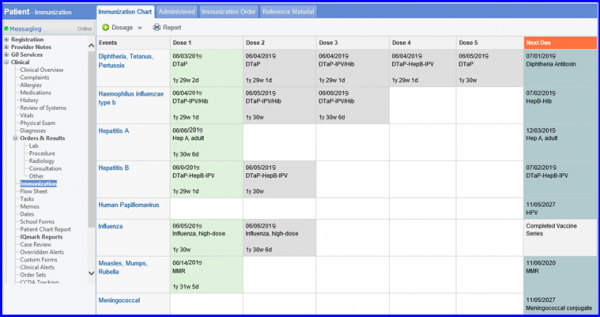
Children who turn 2 (years) during the measurement period and who have a visit during the measurement period. – DENOMINATOR EXCLUSION Exclude patients whose hospice care overlaps the measurement period – NUMERATOR Children who have evidence showing they received recommended vaccines, had documented history of the illness, had a seropositive test result, or had an allergic reaction to the vaccine by their second birthday |
||
| – APPLICATION WORKFLOW
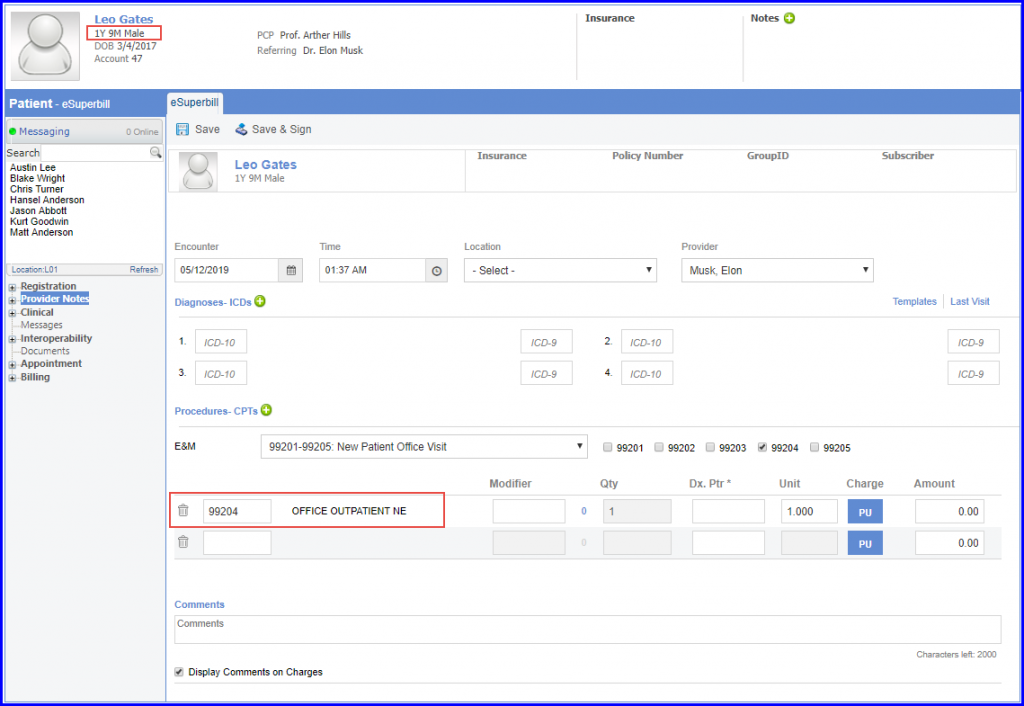
For Denominator: Children who turn 2 year of age during the measurement period and who have a visit during the measurement period are included in this measure. The system captures the Office visit encounters through Visit codes (E&M) which are recorded in either eSuperbills or charges.
For Numerator: Children who have evidence showing they received all of the following criteria are included in the numerator.
To administer recommended vaccines, navigate to Patient > Clinical > Immunization.
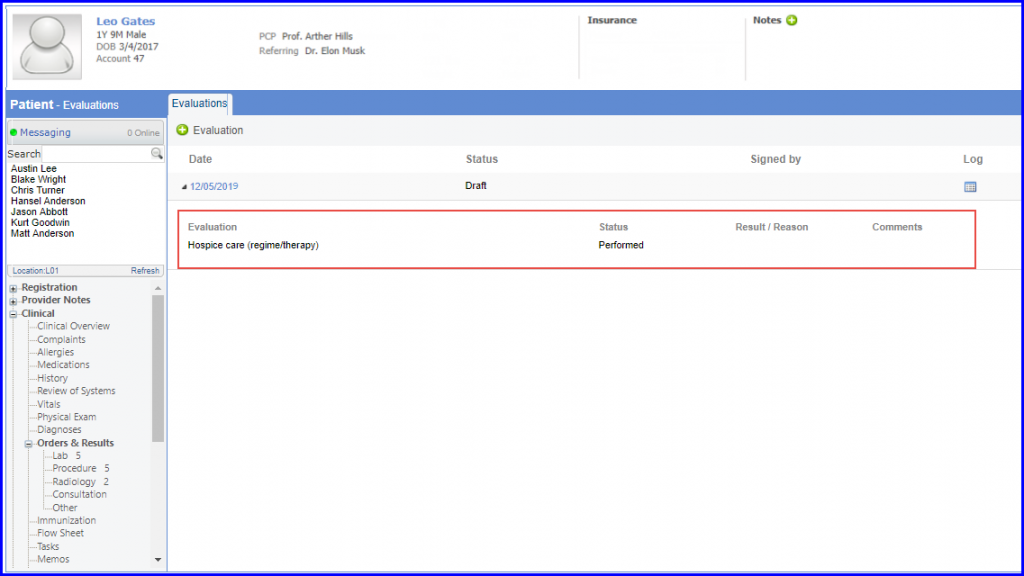
Denominator Exclusion: Patients who were in hospice care during the measurement year are included in denominator exclusion. To document this, navigate to Patient > Clinical > Evaluations and use the below mentioned codes:
|
||