CMS645v3 – Bone Density Evaluation for Patients with Prostate Cancer and Receiving Androgen Deprivation Therapy
| Bone Density Evaluation for Patients with Prostate Cancer and Receiving Androgen Deprivation Therapy | CMS645v3 | All patients determined as having prostate cancer who are currently starting or undergoing androgen deprivation therapy (ADT), for an anticipated period of 12 months or greater (indicated by HCPCS code) and who receive an initial bone density evaluation. The bone density evaluation must be prior to the start of ADT or within 3 months of the start of ADT. |
|---|---|---|
| – DENOMINATOR
Male patients with a diagnosis of prostate cancer and an order for ADT that is intended for greater than or equal to 12 months during the measurement period. Also included are male patients with a diagnosis of prostate cancer with ADT that was administered with an intent of 12 months or greater therapy and began during the measurement period. – DENOMINATOR EXCEPTIONS All patient who refused recommendation for a bone density evaluation after the start of ADT therapy – NUMERATOR All patients with a bone density evaluation within the two years prior to the start of or less than three months after the start of ADT treatment. |
||
| – APPLICATION WORKFLOW
For Denominator: Male patients with a diagnosis of prostate cancer and an order for Androgen Deprivation Therapy (ADT) that is intended for greater than or equal to 12 months during the measurement period are included in denominator. Initial population is calculated through Visit codes. These visits codes are added through eSuperbills or charges. To record an encounter, navigate to Patient > Provider Note > eSuperbill. The documentation of the first encounter takes place when one visit code gets attached with the patient’s eSuperbill/ charge. 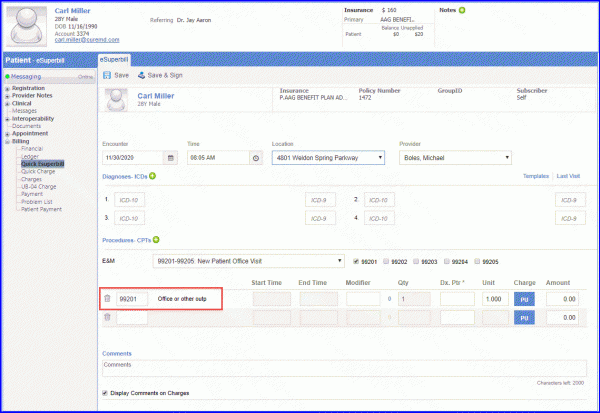 Androgen Deprivation Therapy (ADT) is captured through one of the following workflows:
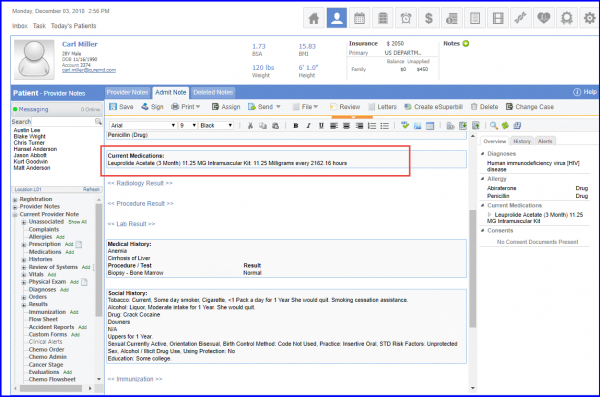
Medication: For documenting active medications for Androgen deprivation therapy for Urology Care, navigate to Patient > Clinical > Medications.
Diagnosis: To add diagnosis of prostate cancer, navigate to Patient > Clinical > Diagnosis.
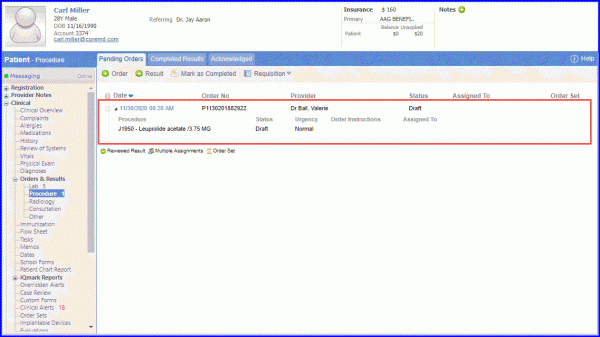
Procedure Order: To record the procedure order of Leuprolide Acetate injection , navigate to Patient > Clinical > Lab and Orders > Procedure Order.
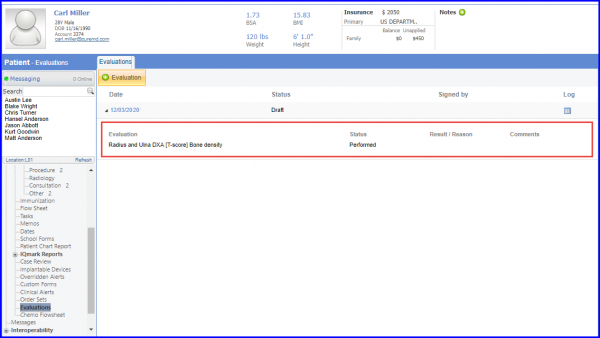
For Numerator: All patients with a bone density evaluation within the two years prior to the start of or less than three months after the start of ADT treatment are included in numerator. To document diagnosis study: density evaluation, navigate to Patient > Clinical > Evaluations.
For Denominator Exceptions: Patient refused recommendation for a bone density evaluation after the start of ADT therapy. Such that no bone density scan is ordered or performed. |
||