CMS153v12 – Chlamydia Screening in Women
| Chlamydia Screening in Women | CMS153v12 | Percentage of women 16-24 years of age who were identified as sexually active at any time during the measurement period and who had at least one test for chlamydia during the measurement period |
|---|---|---|
| – DENOMINATOR Women 16 to 24 years of age by the end of the measurement period who are sexually active and who had a visit in the measurement period. Identifying women as sexually active can be done through:
A qualifying visit in this case can include any of the following:
– NUMERATOR Women with at least one chlamydia test during the measurement period – DENOMINATOR EXCLUSIONS
| ||
APPLICATION WORKFLOW
For Denominator:
- To record an encounter, navigate to Patient > Provider Note > Create Superbill. Under the ‘Procedure- CPTs’ heading, enter the relevant encounter code.
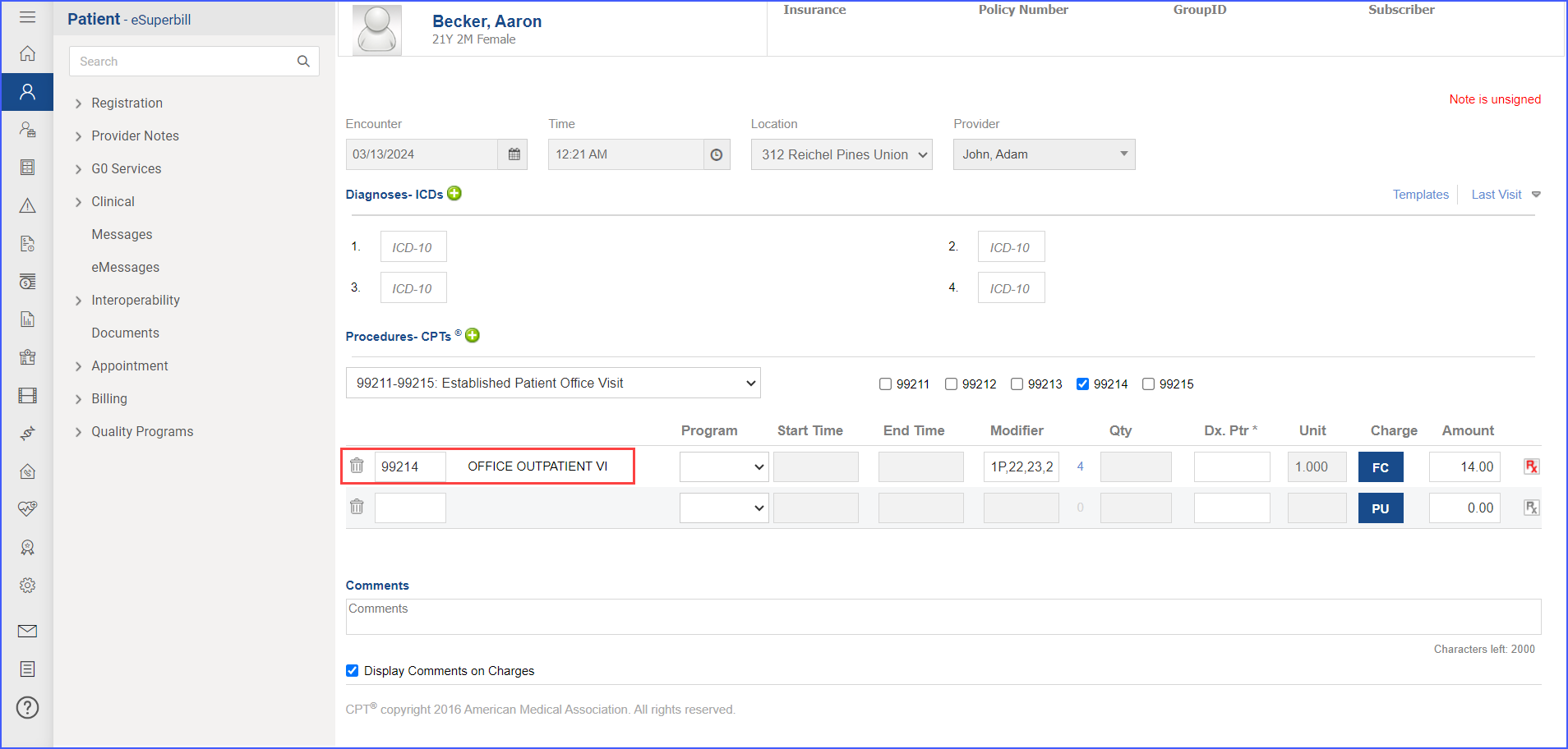
- Sexual activity can be documented through the following methods:
- To document:
- an assessment
- diagnostic study
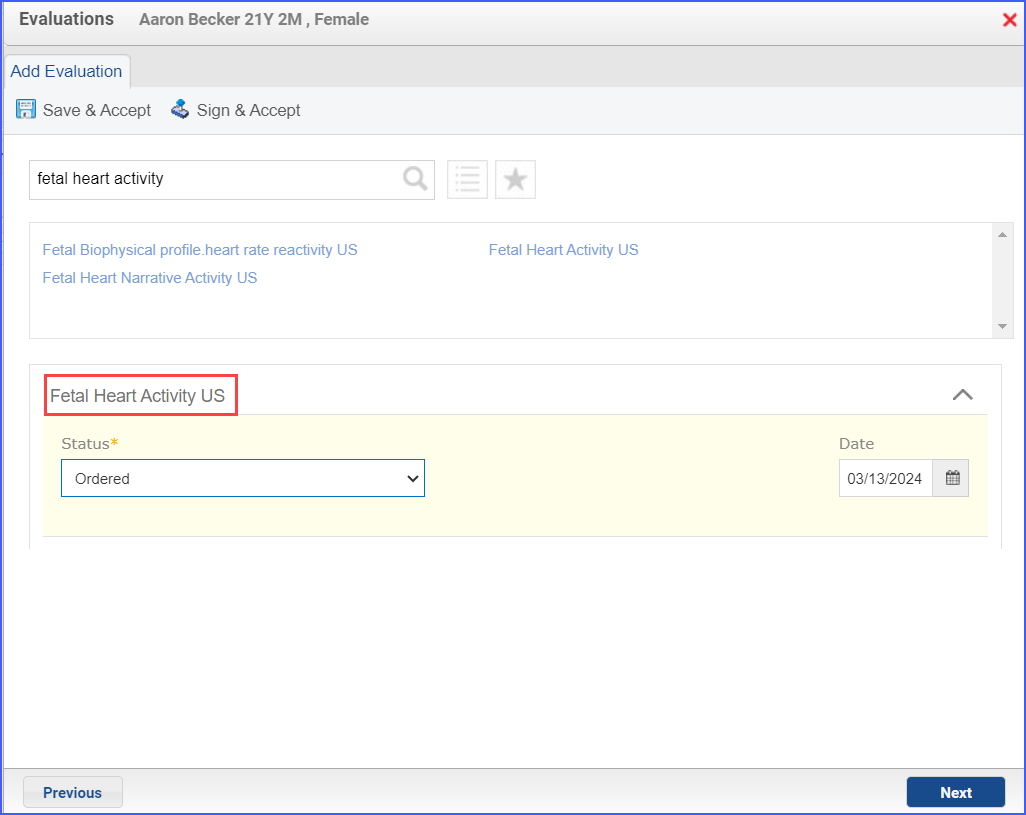
- To document:
navigate to Patient > Provider Note > Evaluations. Click ‘Add’ and search for an assessment or diagnostic study. From the status drop-down select its relevant status and once done, click ‘Save & Accept’ or ‘Sign & Accept’.
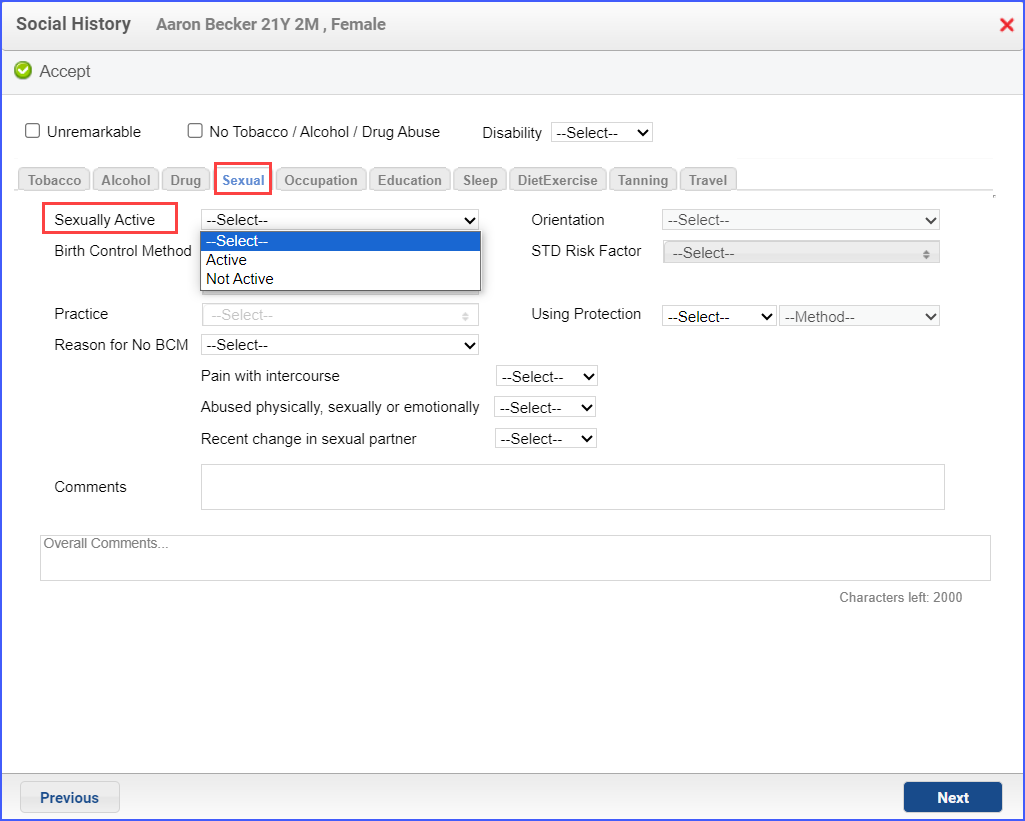
- Sexual activity can also be recorded via the workflow Patient > Provider Note > History > Social > Sexual. Select ‘Active’ from the ‘Sexually Active’ dropdown. Then click ‘Accept’.
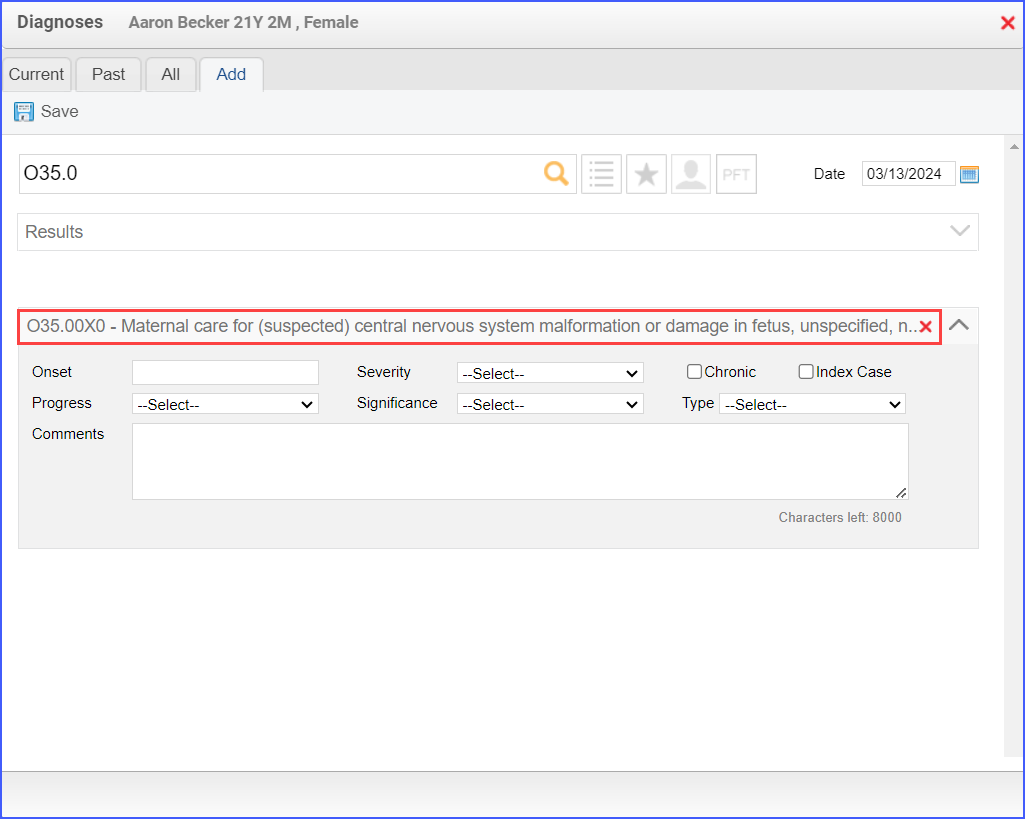
- To document a diagnosis for sexual activity, use the workflow Patient > Provider Note > Diagnoses, and click ‘Add’. Search for the relevant diagnosis and add the diagnosis details. Then click ‘Save’.
- To place an order for a lab test to identify sexual activity, navigate to Patient > Provider Note > Orders > Lab and click ‘Add’. Select a laboratory here and then proceed to search for the relevant lab test. Once done, click ‘Save & Accept’ or ‘Sign & Accept’.
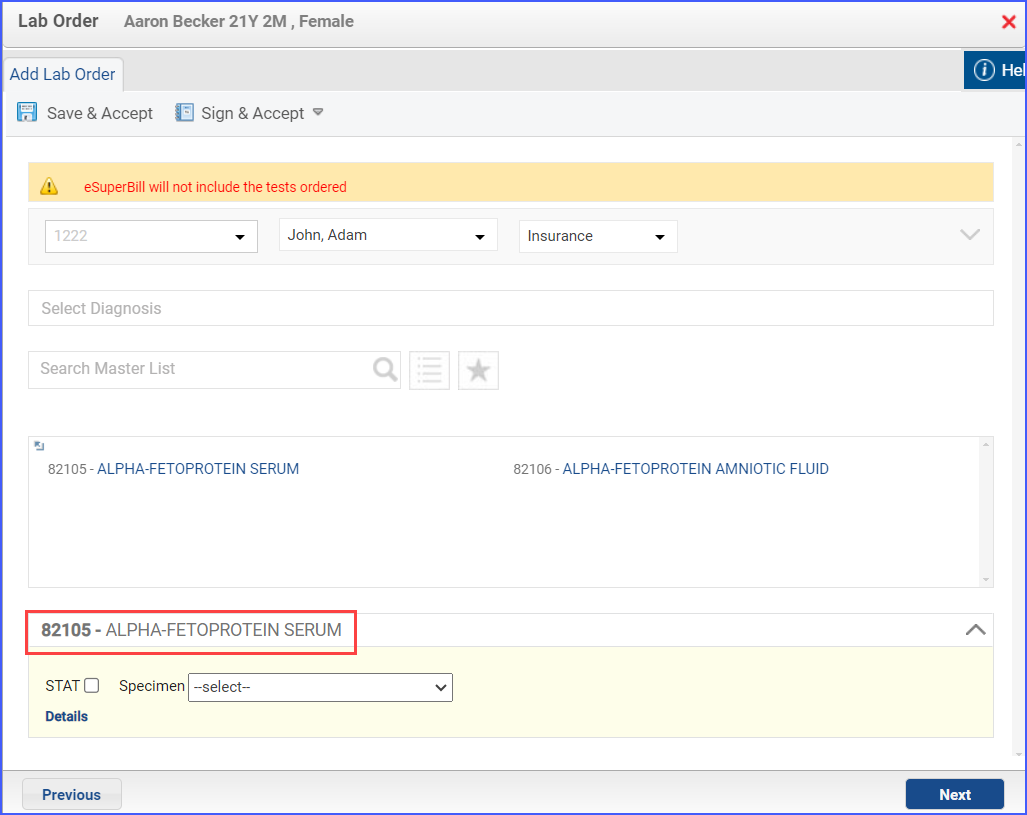
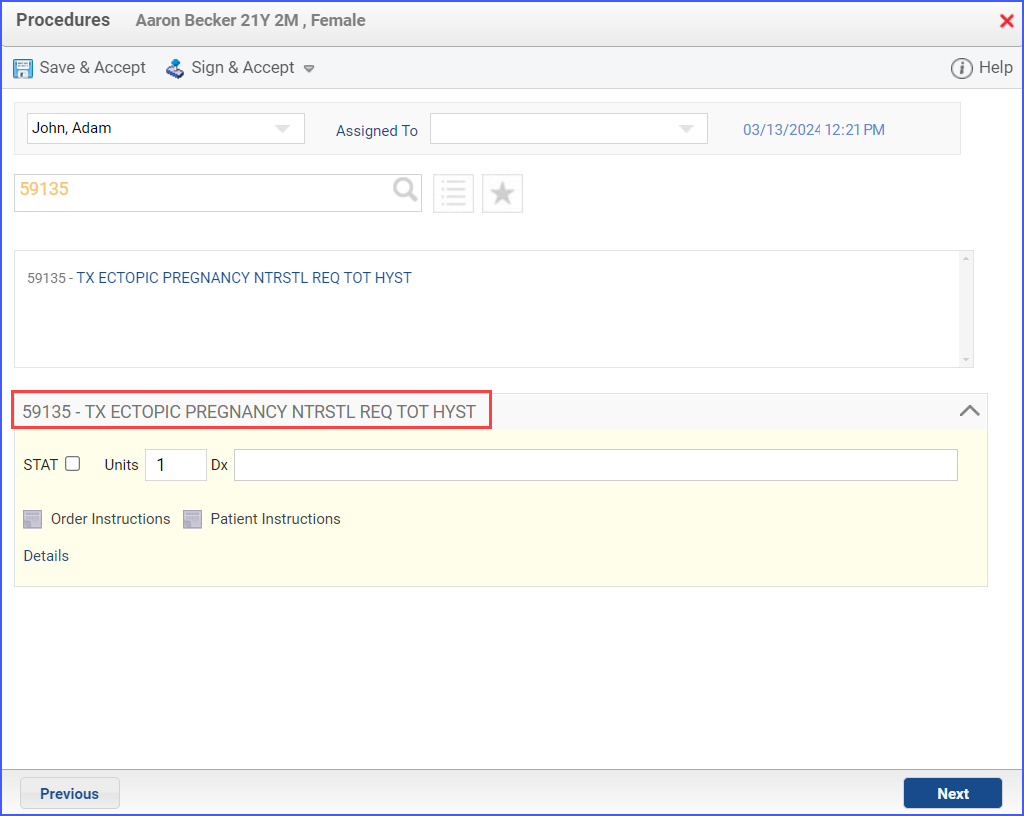
- Procedures identifying sexual activity can be documented through Patient > Provider Note > Orders > Procedures. Here, click ‘Add’ and search for the relevant procedure. Fill out any details needed and once done, click ‘Save & Accept’ or ‘Sign & Accept’.
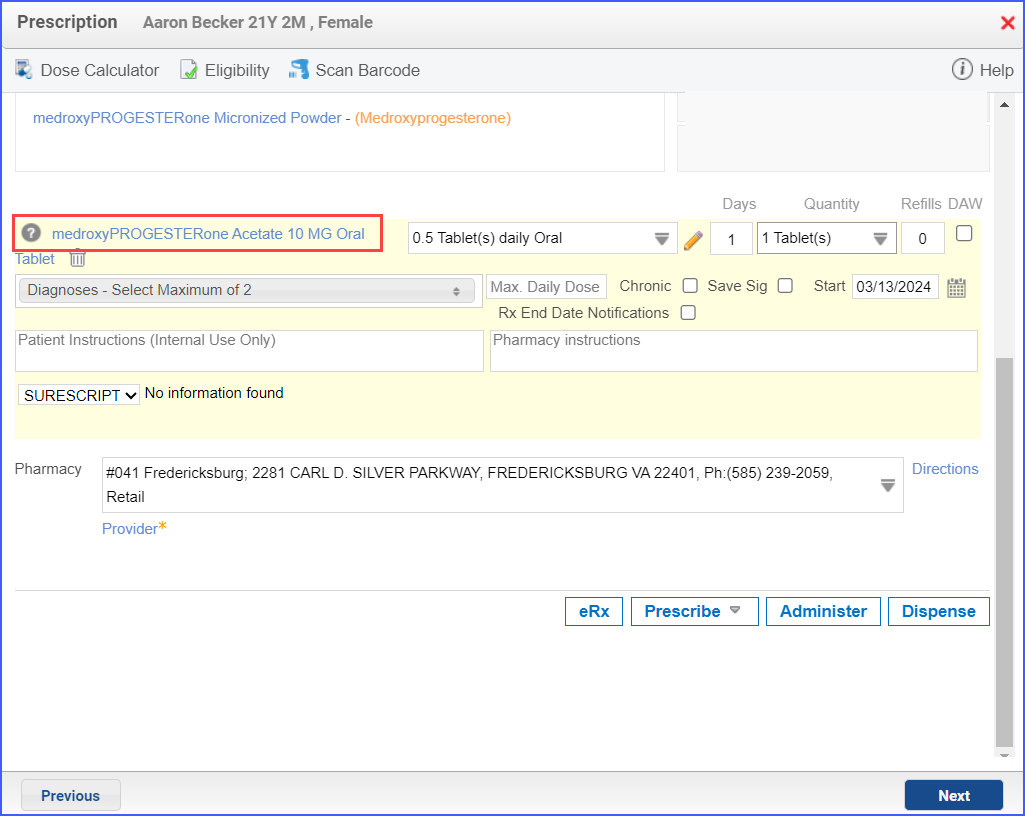
- Contraceptive medication can be ordered through Patient > Provider Note > Prescription while an active contraceptive medication can be documented via Patient > Provider Note > Medications.
For Numerator:
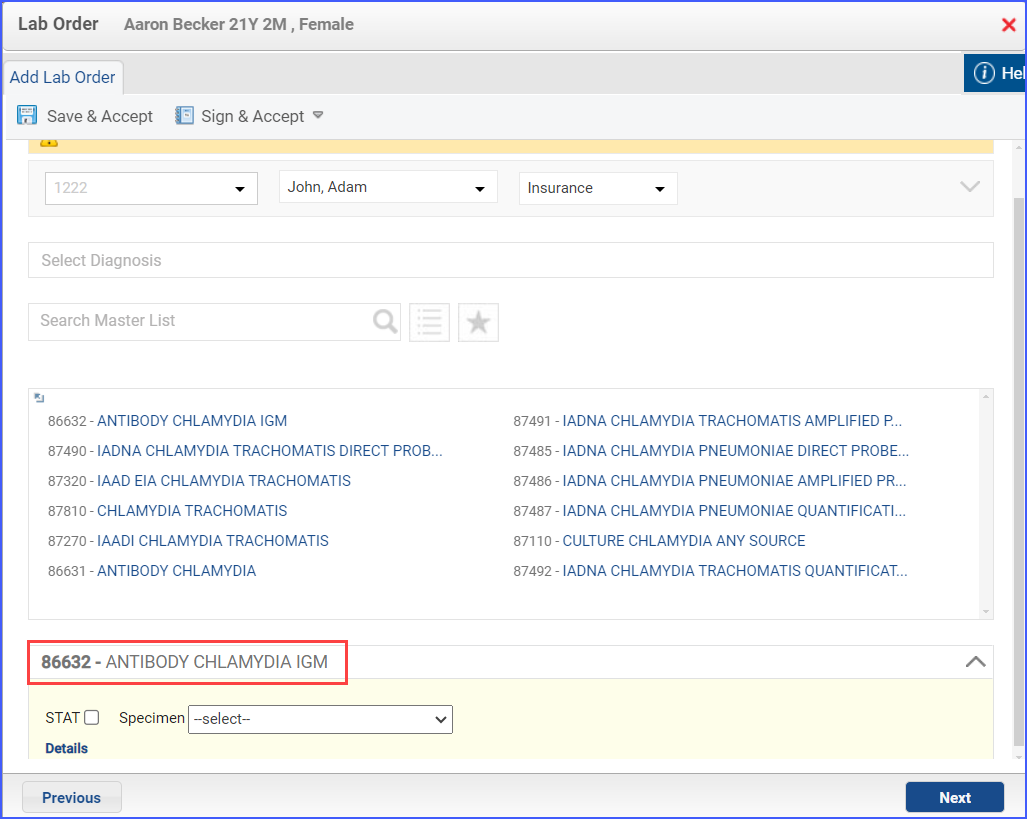
- To order a chlamydia test, use the workflow Patient > Provider Note > Orders > Lab and click ‘Add’. Select a laboratory and from the drop-down menu and then search for the relevant lab test. Once done, click ‘Save & Accept’ or ‘Sign & Accept’.
For Denominator Exclusions:
- To document that a patient is receiving hospice care outside of a hospital or long-term care facility, navigate to Patient > Provider Note > Evaluations. Use the below mentioned codes to order or perform an intervention:
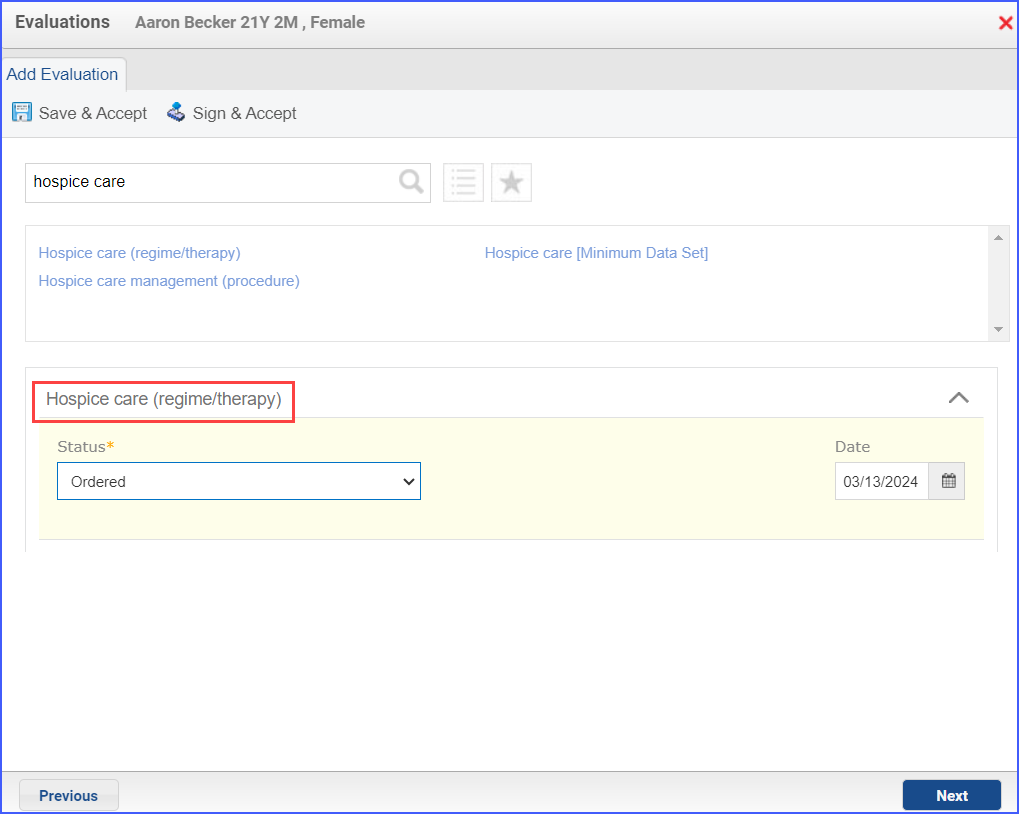
- 385763009: Hospice Care (Regime/Therapy)
- 385765002: Hospice Care Management (Procedure)
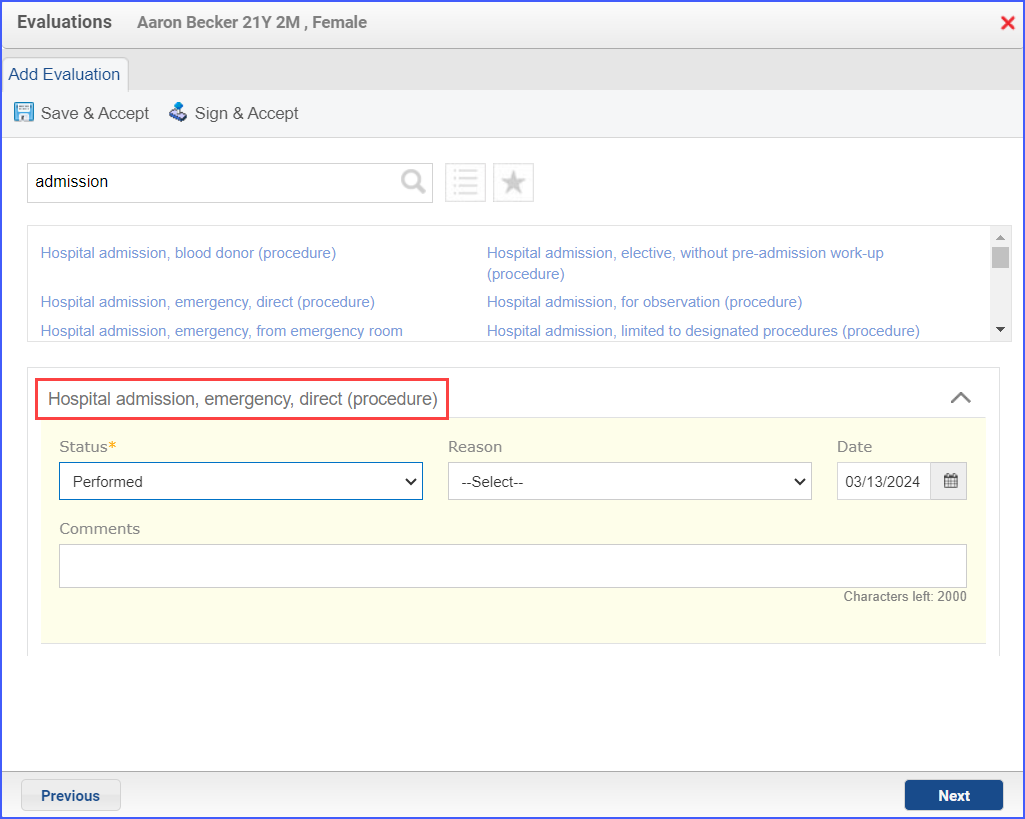
- To document an inpatient encounter, navigate to Patient > Provider Note > Evaluations.
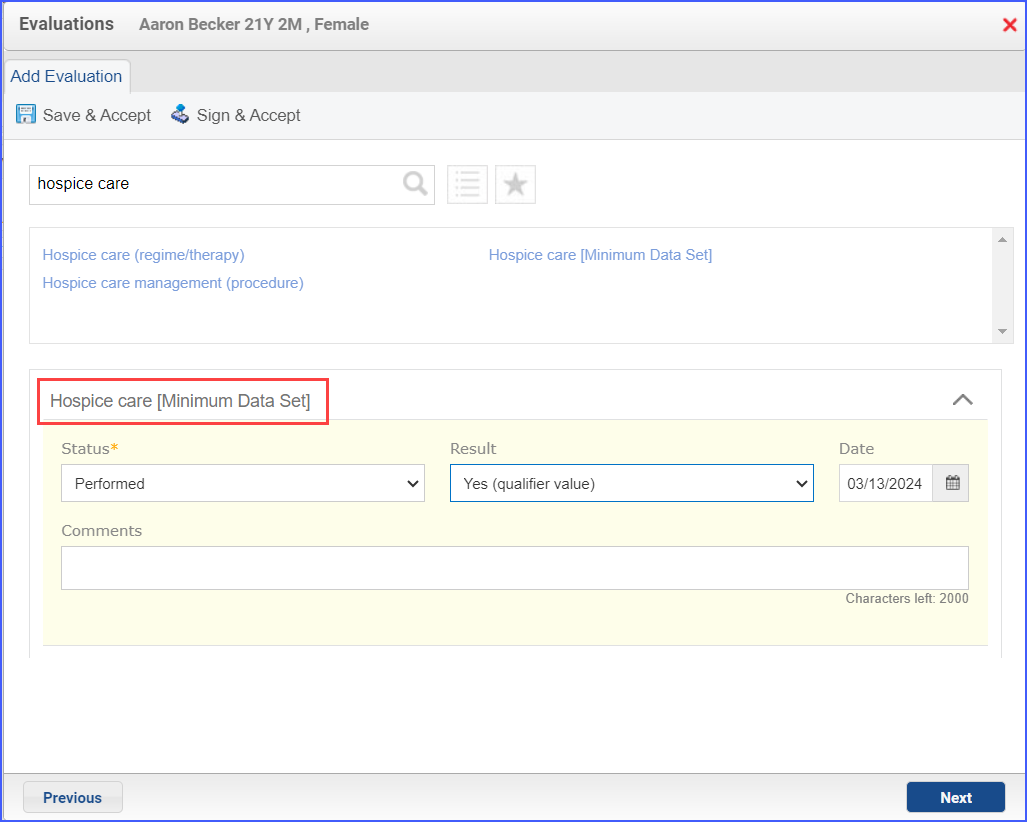
- To document a hospice care assessment, follow the workflow Patient > Provider Note > Evaluations. Click ‘Add’ and search for ‘Hospice Care [Minimum Data Set]’. Then select ‘Performed’ from the ‘Status’ dropdown and ‘Yes’ from the ‘Result’ field.
- A hospice encounter can be documented through Patient > Provider Note > Evaluations or Patient > Provider Note > Create Superbill.
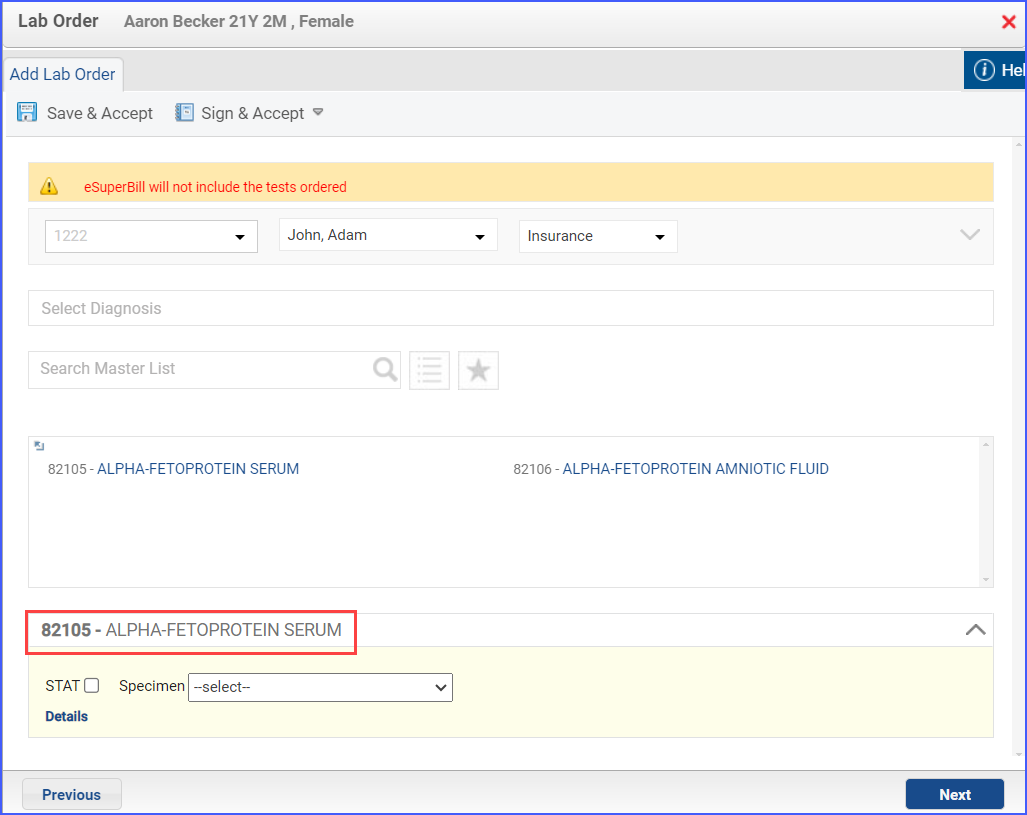
- To place an order for laboratory pregnancy test, use the workflow Patient > Provider Note > Orders > Lab.
Note: The pregnancy test should be ordered during the measurement period.
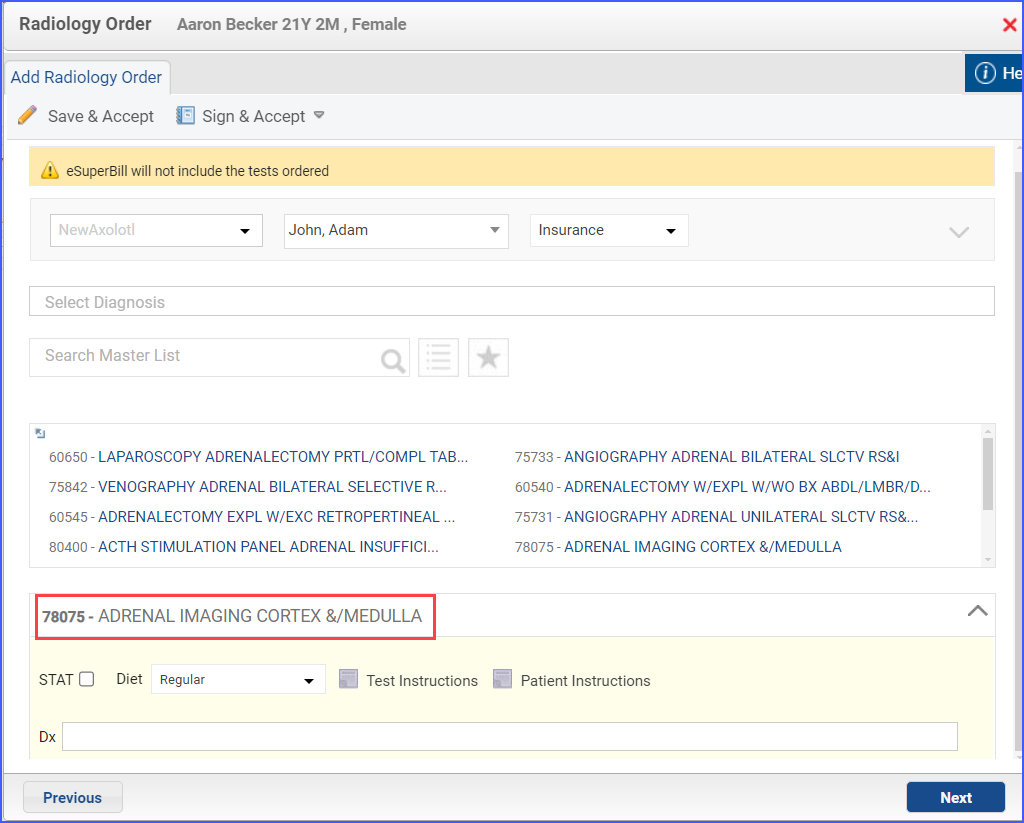
- An ‘X-Ray Study (all inclusive)’ diagnostic study can be ordered via Patient > Provider Note > Orders > Radiology.
Note: ‘X-Ray Study (all inclusive)’ diagnostic study should be ordered 7 days or less after day of ‘Pregnancy Test’.
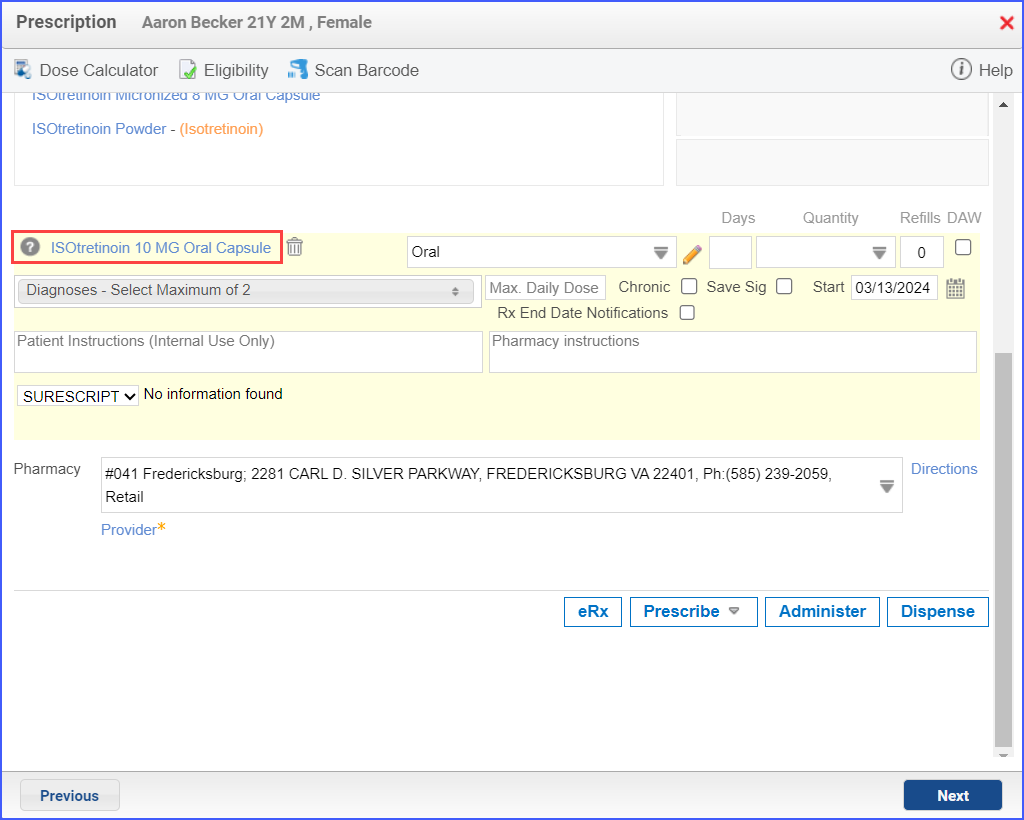
- Isotretinoin can be prescribed using the workflow Patient > Provider Note > Prescription. Here, click ‘Add’ and search for the relevant medication. Fill out any details as needed and once done, click ‘Prescribe’.
Along with the above-mentioned conditions, the following must also be fulfilled in order for females to qualify for denominator exclusions:
- Female patients with no record of diagnoses identifying sexual activity.
- Female patients with no record of active contraceptive medications.
- Female patients with no record of laboratory tests identifying sexual activity but not pregnancy.
- Female patients with no record of procedures identifying sexual activity.
- Female patients with no record of assessments identifying sexual activity.
- Female patients with no record of ordered contraceptive medications.
- Female patients with no record of diagnostic studies identifying sexual activity.
