What is National Drug Code (NDC)?
National Drug Code (NDC) is a unique identifier used for drugs. For drug procedures, NDC code is reported in the claim.
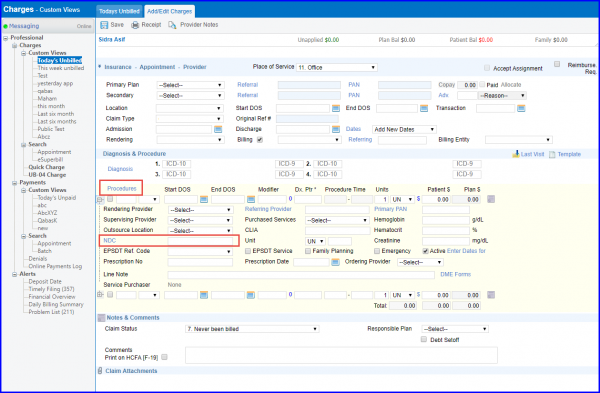
Navigate to “Add/Edit Charges” page following the above mentioned workflow.
The NDC is an eleven digit identifier assigned to a drug product by the labeler/manufacturer under Federal Drug Administration (FDA) regulations. It is comprised of three segments configure in a 5-4-2 format. The format of NDC describes following information:
- Five-digit number uniquely identifies the manufacturing firm and is called Labeler code
- Four-digit number identifies the specific drug, strength and dosage form is called Product Code
- Two digit number identifies package size is called Package Code
User can associate an NDC with a procedure under procedure profile. Once user selects a drug procedure on the charge page, the application automatically loads the NDC.
However, user can change the NDC or add, if not added, under procedure profile. For drug procedures, it is mandatory to add unit information. User can also add Prescription # and Prescription Date with NDC and unit information.