CMS153v10 – Chlamydia Screening for Women
| Chlamydia Screening for Women | CMS153v10 | Percentage of women 16-24 years of age who were identified as sexually active and who had at least one test for chlamydia during the measurement period |
|---|---|---|
| – DENOMINATOR
Women 16 to 24 years of age who are sexually active and who had a visit in the measurement period – NUMERATOR Women with at least one chlamydia test during the measurement period – DENOMINATOR EXCLUSIONS
|
||
| – APPLICATION WORKFLOW
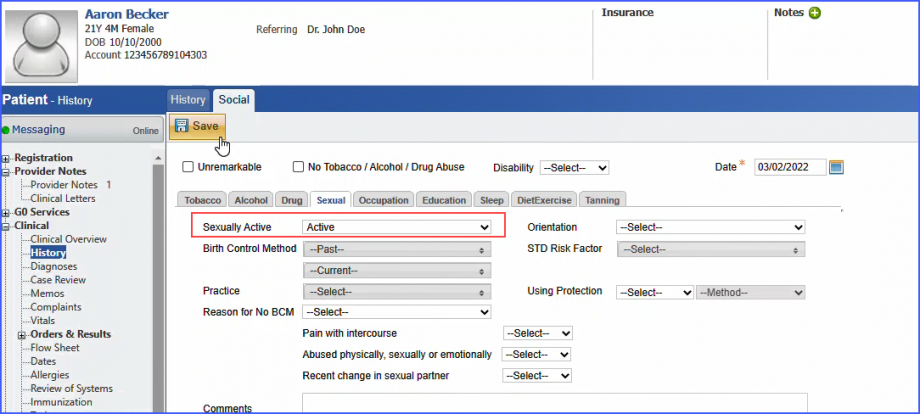
For Denominator: Include female patients aged 16 – 24 at the start of the measurement period with a qualifying encounter during the measurement period. To record an encounter, navigate to Patient > Provider Note > eSuperbill. Under the ‘Procedure- CPTs’ heading, enter the encounter code. 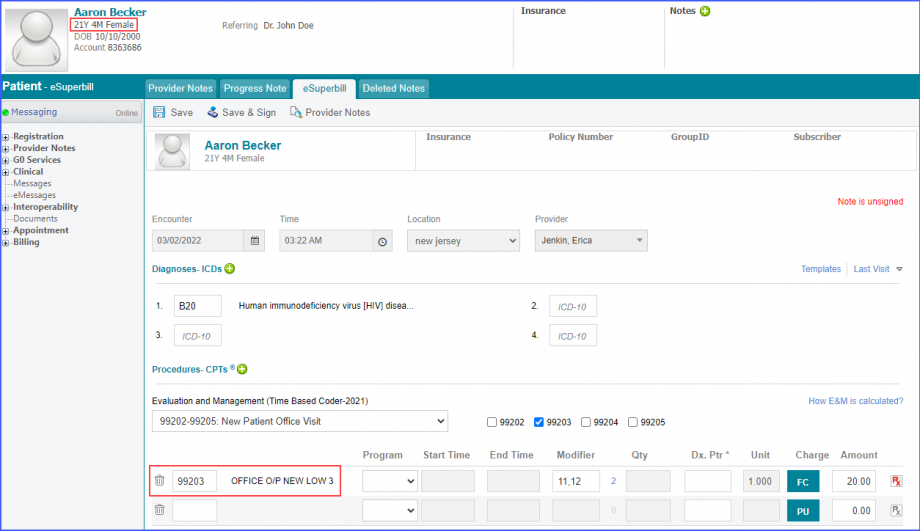 Additionally, female patients should fulfill one of the following criteria during the measurement period:
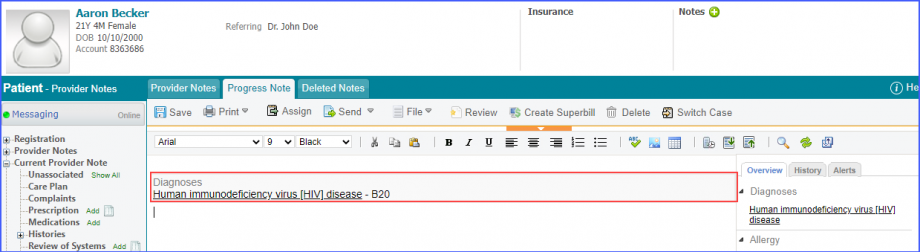
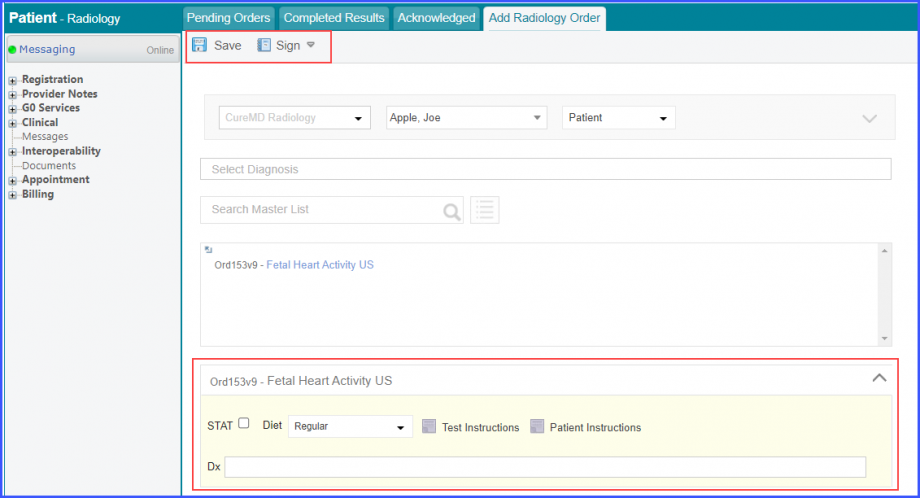 Mark the radiology order as completed by clicking the ‘Mark As Completed’ button. 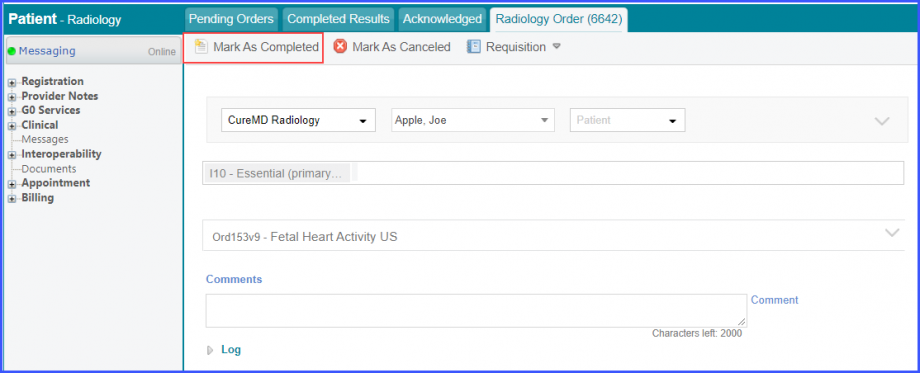 Note: For LOINC Codes Based Labs: Please ensure that the test code is mapped to a LOINC Code from the Settings. To map a LOINC with the radiology test code, navigate to Settings > EHR > Radiology. Select the radiology test and navigate to the ‘Code’ tab. Then, click on a code. Under the ‘Result Codes and LOINCS’ heading, add a Code, LOINC and Description and click on the ‘Add’ button. Once done, click on ‘Save’.  For CPT Codes Based Labs: When a CPT code is added to a test order, please ensure that the CPT code is present in the ‘Procedures’. 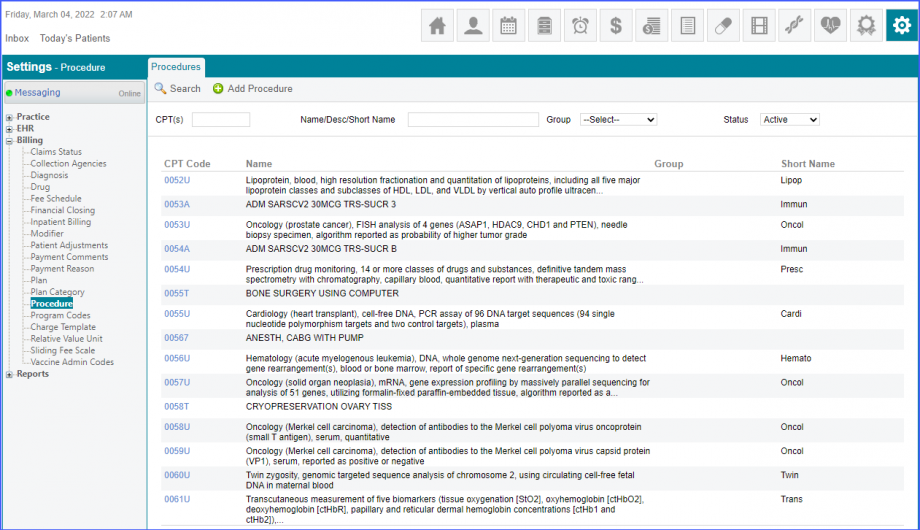 Moreover, the CPT code should be mapped to a LOINC Code from the Settings. To map a LOINC with the CPT code, navigate to Settings > Billing > Procedure. Select the CPT code and navigate to the ‘Edit Procedure’ tab. Under the ‘Procedure Components’ heading, add a Code, LOINC and Description and click on the ‘Add’ button. Once done, click on ‘Save’. 
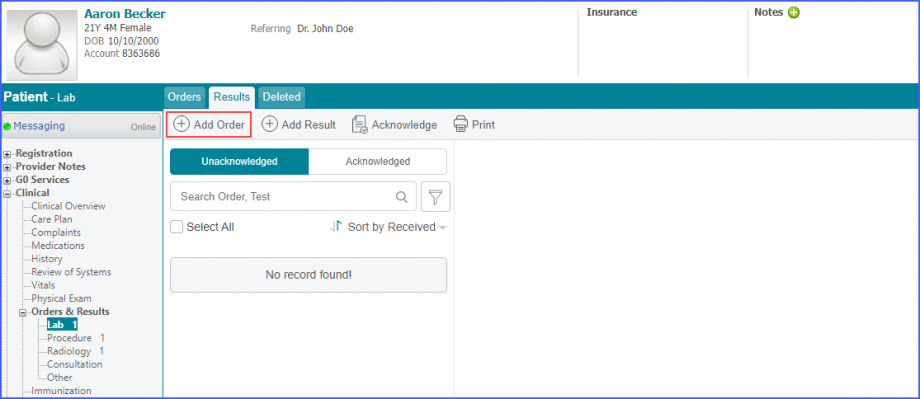 Here, select a laboratory and search for the lab test. Once done, click on the ‘Save’ or ‘Sign’ button. 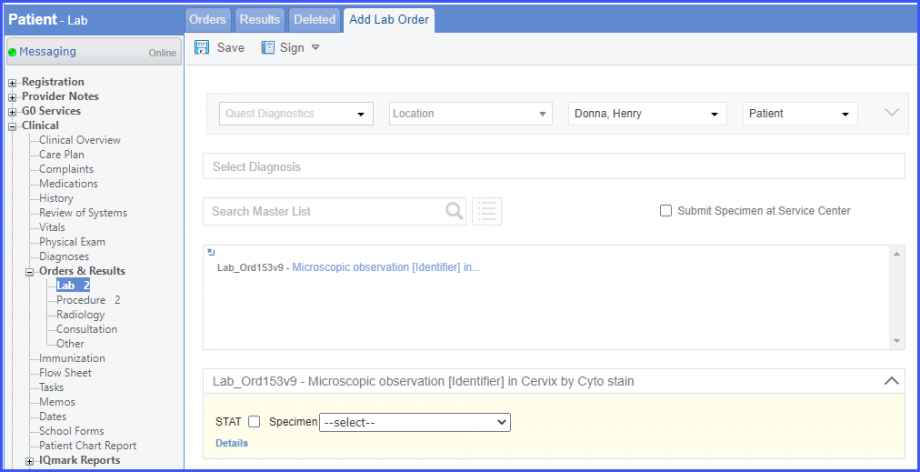 Next, click on the ‘Mark As Received’ button. 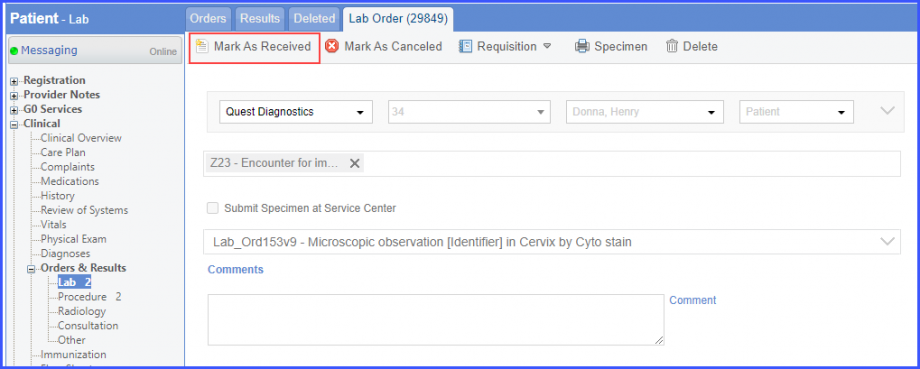 On the ‘Edit Lab Result’ screen, add the observations and click on the ‘Save’ button. 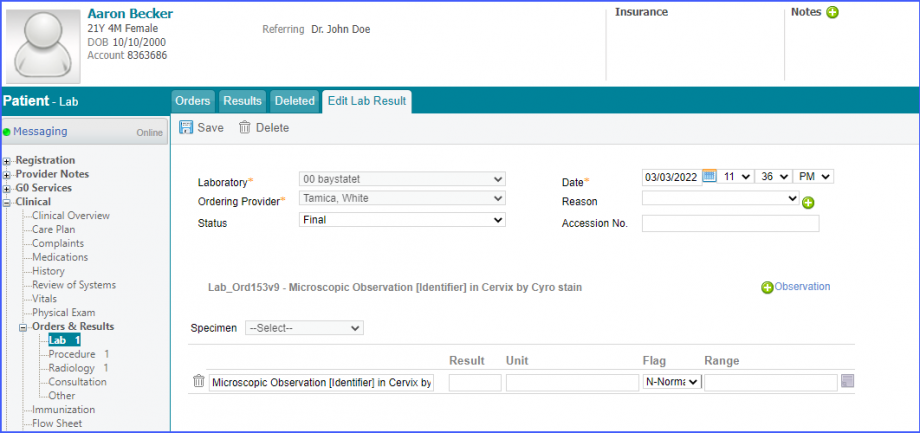 Note: For LOINC Codes Based Labs: Please ensure that the lab code is mapped to a LOINC Code from the Settings. To map a LOINC with the lab code, navigate to Settings > EHR > Laboratory. Select the Lab and navigate to the ‘Code’ tab. Then, click on the Lab Order. Under the ‘Result Codes and LOINCS’ heading, add a Code, LOINC and Description and click on the ‘Add’ button. Once done, click on ‘Save’. 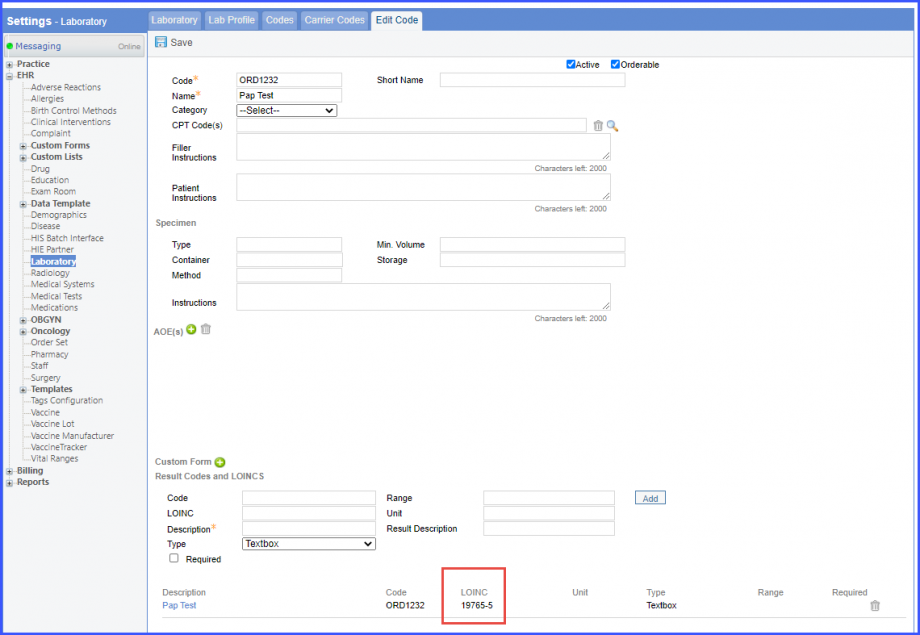 For CPT Codes Based Labs: When a CPT code is added to a lab order, please ensure that the CPT code is present in the ‘Procedures’. 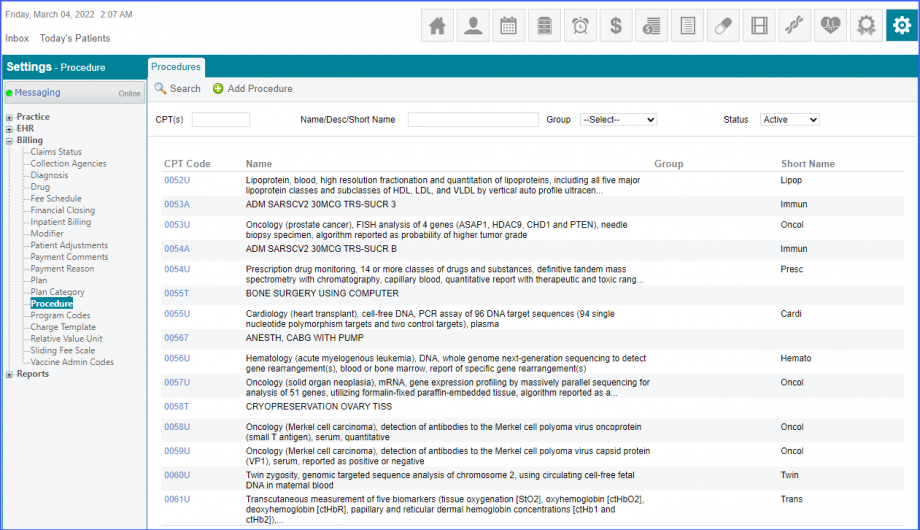 Moreover, the CPT code should be mapped to a LOINC Code from the Settings. To map a LOINC with the CPT code, navigate to Settings > Billing > Procedure. Select the CPT code and navigate to the ‘Edit Procedure’ tab. Under the ‘Procedure Components’ heading, add a Code, LOINC and Description and click on the ‘Add’ button. Once done, click on ‘Save’. 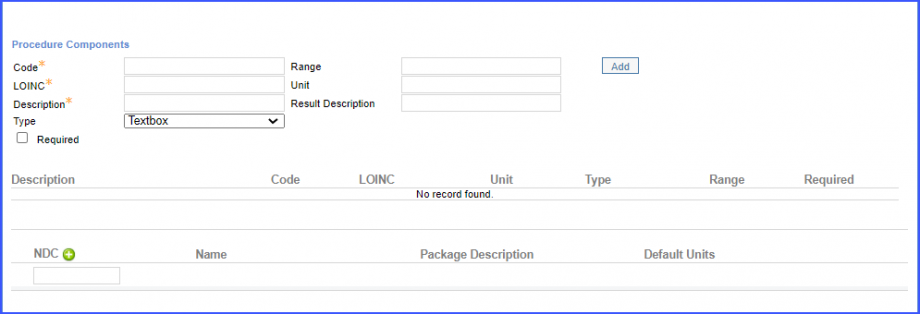
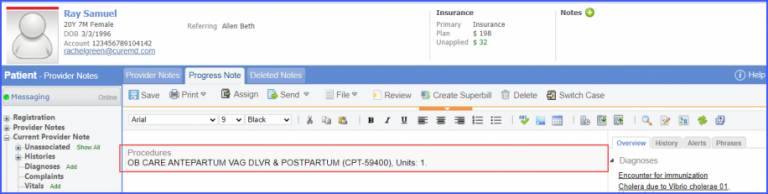 Note: Please ensure that the Procedure is present in the system. For this, navigate to Settings > Billing > Procedure. If the procedure is not added then, click on the ‘Add Procedure’ button. 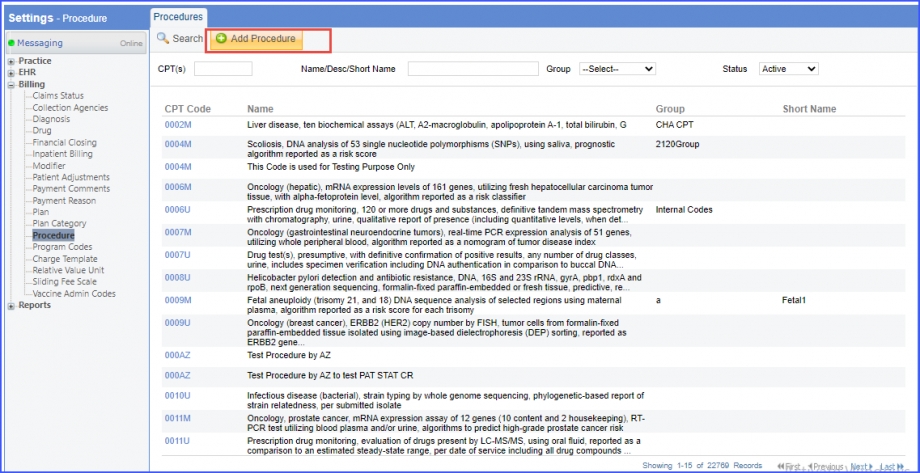 Here, enter the ‘Code’ and ‘Name’ of the Procedure. Once done, click on the ‘Save’ button. 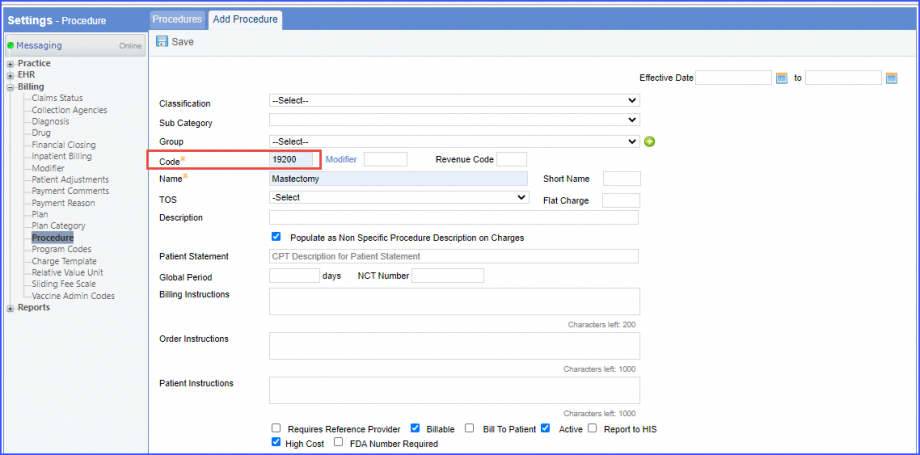
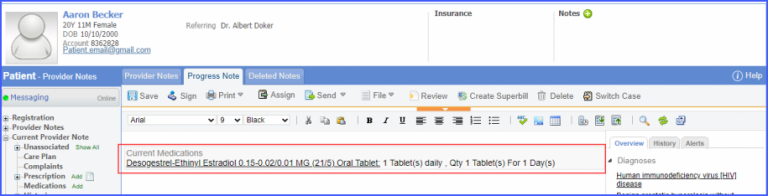
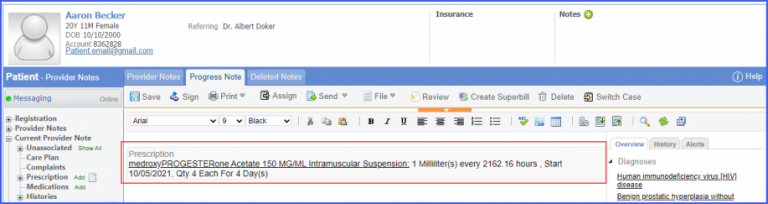 For Numerator: Include female patients with ‘Chlamydia Screening’ lab test performed during the measurement period. The test result must not be null. To record a lab test, navigate to Patient > Clinical > Orders&Results > Labs and click ‘+ Add Order’ button. Then select a laboratory and search for the lab test. Once done, click on the ‘Save’ or ‘Sign’ button. Next, click on the ‘Mark As Received’ button. On the ‘Edit Lab Result’ screen, add the observations and click on the ‘Save’ button. For Denominator Exclusions: Exclude patients satisfying any of the following conditions:
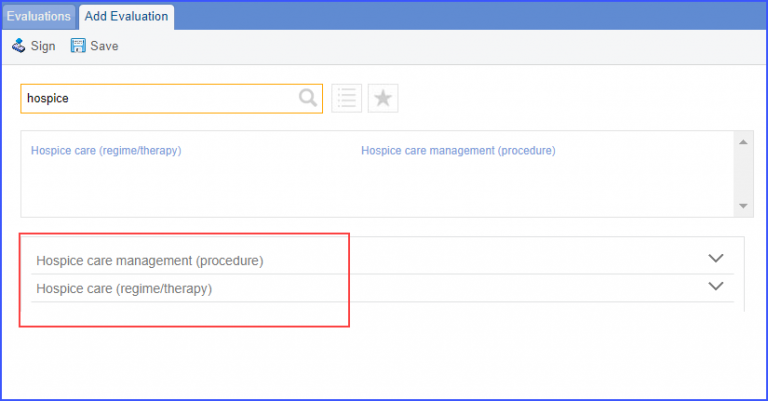
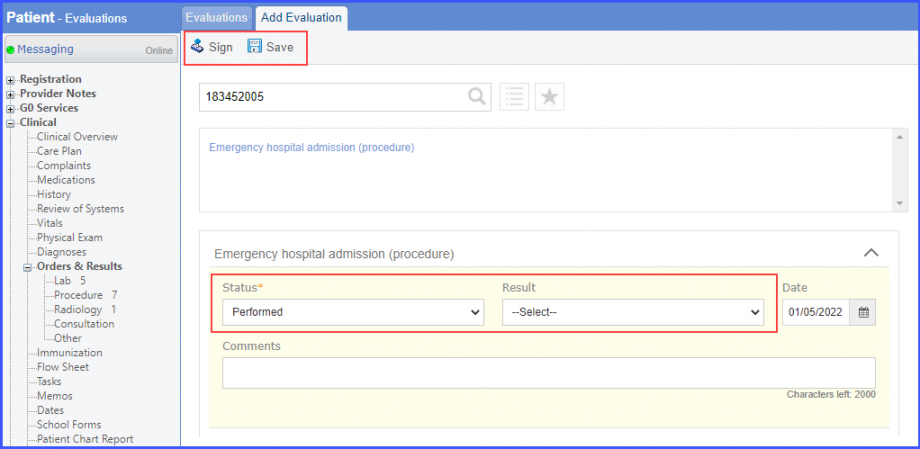
|
||
